

Yet, based on what appears to be a potential problem, investigation is warranted. 4, 5 As is the case with all voluntary adverse event reporting to the FDA, data may be incomplete, causality is not definitive, under- or over-reporting may exist, and the number of reports does not indicate incidence. In the November 2012, the FDA announced an ongoing investigation based on recent reports of significant injury or death associated with products marketed as “energy drinks.” 3 Summarized data from voluntary reports received by the FDA from Januthrough Octorevealed that adverse events ranged from nonserious (eg, nausea, vomiting, anxiety, and flushing) to significant or serious (eg, renal failure, seizures, arrhythmias, or death). However, the role of co-ingredients as risk factors or confounders has not been established. 2 Some of the reported adverse effects associated with energy drinks are known reactions to caffeine (eg, anxiety, nausea).
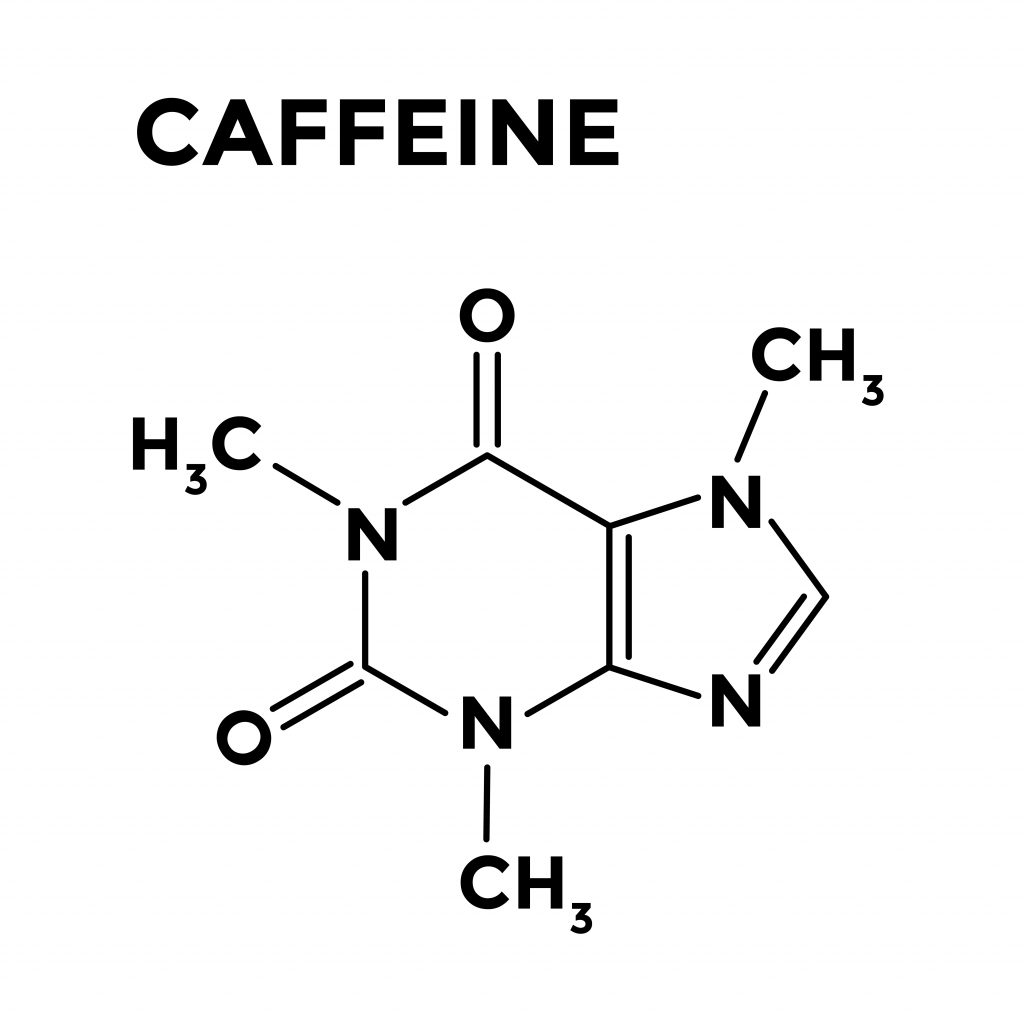

1 In energy products, the caffeine content varies greatly, from 47 mg to 80 mg per 8 ounces to as high as 207 mg per 2 ounces. 1 The caffeine content in a typical 5 ounce cup of coffee ranges from 60 to 100 mg. Although commonly viewed as beverages or food products by consumers, the primary ingredient, caffeine, is considered both a food additive and a drug by the US Food and Drug Administration (FDA). Recently, energy drinks/products have enjoyed increased popularity.


 0 kommentar(er)
0 kommentar(er)
